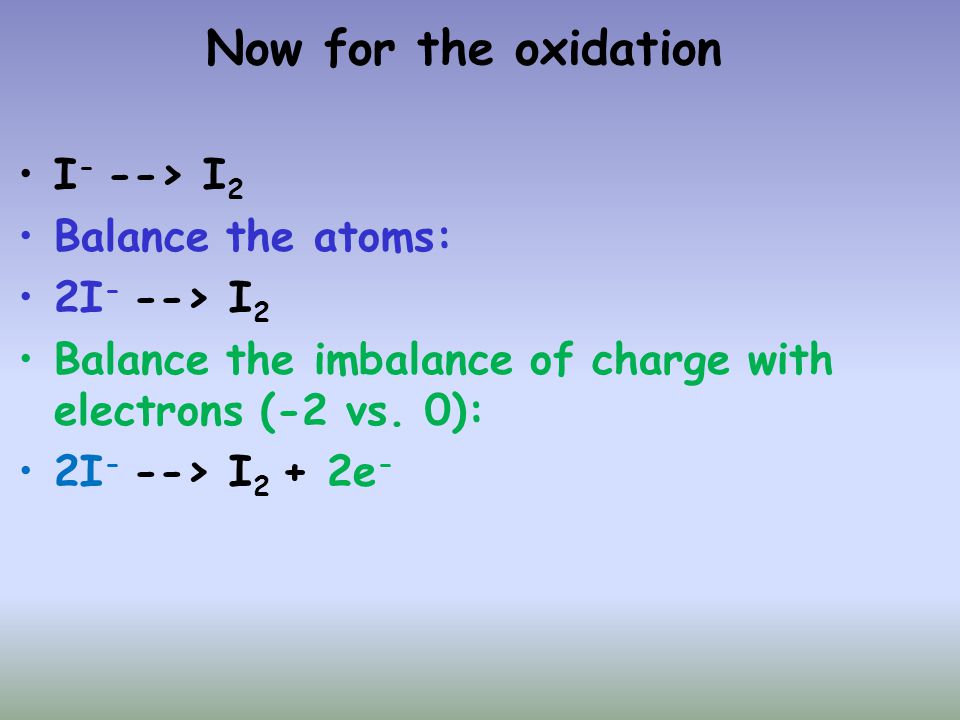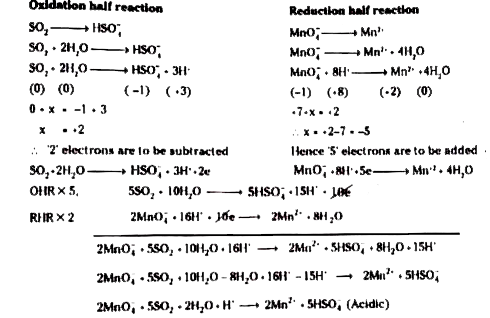MnO4 + I = MnO2 + I2 balance this equation by oxidation method in basic medium and give all the steps - Brainly.in

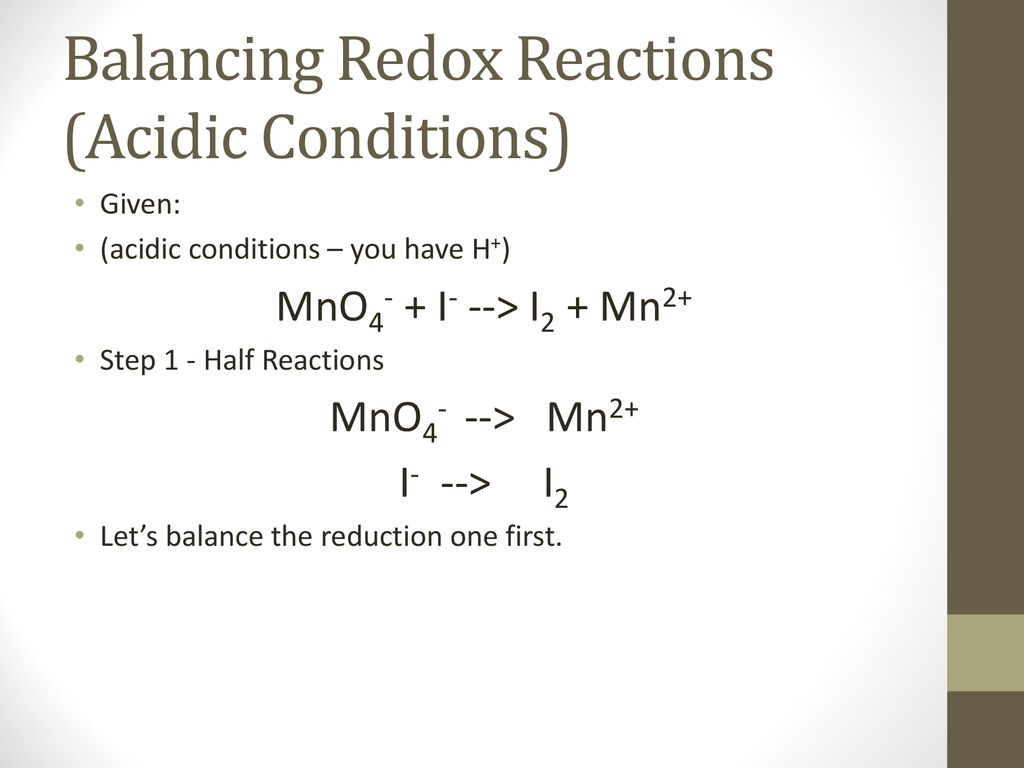
Balance the following redox reactions by the ion - electron method in basis medium MnO^-4(aq) + I^-(aq)→ MnO2(s) + I2(s)

Balance the following redox reactions by the ion - electron method in basis medium MnO^-4(aq) + I^-(aq)→ MnO2(s) + I2(s)

SOLVED: 4. Write balanced equations for the following redox reactions:a. NaBr + Cl2 NaCl + Br2b. Fe2O3 + CO Fe + CO2 in acidic solutionc. CO + I2O5 CO2 + I2 in

Hasbro Beyblade Burst - Evolution Single Pack - Ifritor I2 (Balance) - Right Spin Battle Top - Ages 8+ : Amazon.com.au: Toys & Games
Balance the following ionic equations (i) Cr2O7^2-+H^++I^- → Cr^3+ +I2+H2O - Sarthaks eConnect | Largest Online Education Community